HIV self-testing
HIV self-testing is a testing option that was licensed for use in Canada in November 2020. It has been available in several other countries around the world for many years. With a self-test, an individual collects their own sample, conducts the test and interprets the result themselves. The self-test that is available in Canada uses a blood sample from a finger prick. It is a screening test, meaning that a reactive (positive) result needs to be confirmed by a laboratory HIV test.
What is the benefit of self-testing?
Self-testing complements testing that is offered in medical and community settings, and it has the potential to increase the overall uptake of testing. Self-testing may be particularly useful for reaching people who have never tested before and those who are not tested as often as guidelines recommend. Many studies have shown that self-testing is an acceptable option to people in priority populations for HIV testing.
What technology is used for HIV self-testing?
The only self-test that is currently licensed in Canada is the INSTI HIV Self Test. It uses the same technology as the rapid point-of-care (POC) INSTI HIV-1/HIV-2 Antibody Test, which individuals can get from a healthcare provider or community worker in some parts of Canada. It is a screening test done with a blood sample from a finger prick. A person can do the test alone or have someone else (such as a friend, partner or family member) present when they take the test.
How does the test detect HIV and how soon after a potential exposure will the test work?
The self-test works by detecting HIV antibodies, which are proteins produced by the body’s immune system in response to HIV infection. It can take between three and 12 weeks for the test to be able to detect antibodies from the time a person was exposed to HIV. In 50% of people, antibodies can be detected by about 22 days after exposure to HIV and in 99% of people they can be detected by 12 weeks after exposure.
The period of time between when a person has been exposed to HIV and when a test can tell they have HIV is called the window period. The length of the window period varies from person to person. Some people develop antibodies that are detected by HIV tests slowly and some people develop them more rapidly. Once these antibodies are present in amounts that the test can detect, the window period is over. Therefore, if at any time a person gets a reactive result on a self-test, they probably have HIV and should get a confirmatory test.
If someone has had a recent exposure to HIV and gets tested for HIV during the window period, the test may come back as negative (non-reactive) even though the person actually has HIV. This would happen if their body has not started producing antibodies at levels that are detectable by a test. When a test result is negative after a recent exposure to HIV, the person should be retested three months from their last potential exposure to confirm they are HIV negative. Some provinces recommend testing at intervals until the end of the window period to pick up HIV infection as early as possible.
Since self-tests detect antibodies only, they have a longer window period than standard laboratory tests that also detect the p24 antigen (a part of the virus itself).
What does a self-test kit look like?
A testing kit contains instructions and everything needed to take the test:
| A testing device |  |
| Three small bottles |    |
| A bandage |  |
| A sterile single-use lancet |  |
The instructions include the steps to take the test, information that a person should know before and after taking the test, and links to useful community services and supports. The instructions also include a link to a self-test demo video that people can watch before they take the test.
What are the steps for a person to take the test?
Before a person takes a test, they should read the instructions in detail.
To take a self-test, the tester must:
- Wash and dry their hands.
- Use the lancet to draw a drop of blood and allow the drop of blood to fall into bottle 1, without touching their finger on the opening of the bottle. Cap the bottle.
- Shake and pour the liquid from this bottle into the testing device and allow a few seconds for the liquid to disappear.
- Shake and pour the liquid from bottle 2 into the device and allow a few seconds for the liquid to disappear.
- Shake and pour the liquid from bottle 3 into the device and allow a few seconds for the liquid to disappear.
In total, the test normally takes less than five minutes to perform.
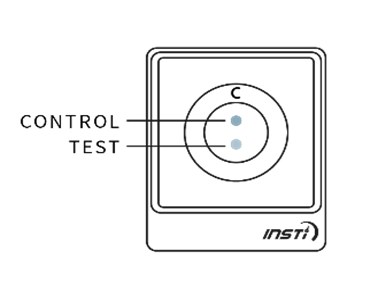
The results of the test will be displayed on the testing device as soon as the liquid from bottle 3 disappears into the device. The test results are simple to interpret. A control dot should appear near the top of the test window to indicate that the test has worked. If no control dot appears, the test kit has not worked and the test should be repeated using a new kit.
If a second dot appears (below the control dot), this indicates a reactive result. If no second dot appears, this indicates a negative result.
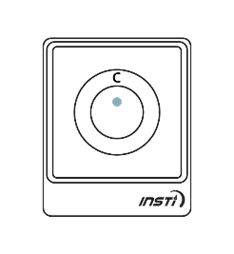
The following images show the possible results of the test:
| Reactive result (positive) | Non-reactive result (negative) | Invalid test |
|
One dot may be lighter than the other. In rare instances, a faint ring may appear at the test dot; this is a positive result. |
 |
  |
What happens after a non-reactive (negative) HIV self-test result?
No further testing is done to confirm a negative result. However, if a person has had a potential exposure to HIV within the three-month window period of the test, the person should take another HIV test later or go to a healthcare provider for a laboratory HIV test that has a shorter window period.
If a person tests negative and is at ongoing risk for HIV, they should retest regularly. They can also consider taking pre-exposure prophylaxis (PrEP) to help prevent HIV.
What happens after a reactive (positive) HIV self-test result?
After someone receives a reactive HIV self-test result, it means that the person probably has HIV but this needs to be confirmed with a laboratory-based confirmatory test. The individual will need to go to a healthcare provider for confirmatory testing.
If the confirmatory test returns a positive result, an HIV diagnosis is made. As with any confirmed HIV positive diagnosis, this initiates a series of processes including post-test counselling, Public Health notification, partner notification and linkage to care. See CATIE’s fact sheet on the HIV testing process for more information.
How accurate is the INSTI HIV Self Test?
The INSTI HIV Self Test is very accurate. The accuracy of an HIV test is measured by its sensitivity and specificity. Sensitivity is the chance that a reactive test result will correctly indicate that a person has HIV. In other words, if the person has HIV, the test will detect it. Higher sensitivity means there is a lower chance of a false-negative result (i.e., a negative test result for a person who is actually HIV positive). Specificity is the chance that a negative test result will correctly indicate that a person does not have HIV. In other words, if the person does not have HIV, the test will be negative. Higher specificity means there is a lower chance of a false-positive result (i.e., a positive result for a person who is actually HIV negative).
The INSTI Rapid Self Test has a sensitivity of 99.6%. In other words, if 1,000 HIV-positive people were tested for HIV, four of them might incorrectly test negative. Since the vast majority of people who get tested for HIV are actually HIV negative, the chance of a negative result being false is extremely low.
The specificity of this test is slightly lower, at 99.3%. In other words, if 1,000 HIV-negative people were tested, seven of them might incorrectly test positive. Therefore, the chance of false positives is extremely low, but it is slightly higher than the chance of false negatives. This is why all people with reactive test results are sent for a confirmatory test, which has a specificity of 100%. This means that the chance of a false-positive result after confirmatory testing is essentially zero.
Are self-tests easy for people to use correctly?
Research shows that the majority of people are able to use self-tests correctly. A review article looked at studies that examined what percentage of people who took a self-test were able to achieve the same result as when a healthcare provider performed the test on them. In nine studies that looked at this for blood-based self-tests, the agreement between the self-test result and the result from the test done by a healthcare provider ranged from 85.4% to 100%. In seven of these nine studies, the agreement was 97% or higher.
When self-testers aren’t able to achieve the same result as a healthcare provider, it is usually because the person taking the test made an error, such as not catching the drop of blood in the bottle. Testers may also make an error when interpreting the results. To avoid such errors, it is important for people to read the instructions carefully before taking the test. Testers who have a language barrier that prevents them from understanding the instructions may require support.
Are self-tests acceptable to people?
Research shows that self-testing is an acceptable option for a large proportion of people. A review paper looked at the acceptability of self-testing in 14 studies. Acceptability was defined either as indicating a willingness to take a self-test or as an increase in testing frequency as a result of using a self-test. In eight of the 14 studies, self-testing was acceptable to at least two-thirds of participants.
In a Canadian study of self-testers, roughly 95% of the survey respondents said that they would use a self-test again and would recommend it to a partner or friend.
There are a number of reasons why an individual might find the option of self-testing appealing. Self-testing can be a good option for people who are reluctant to get a test because of stigma (e.g., people who have had negative experiences with healthcare providers, or those who worry about being seen going into a testing clinic). The anonymous nature of self-testing may also be appealing to people who have confidentiality concerns and do not want to have a test result associated with their medical records. Finally, self-testing may be more convenient for some people, because it does not require them to go to a medical facility or testing clinic.
Where can someone get a self-test?
Several community organizations and research projects offer free self-test kits to people in Canada. Free kits can be ordered online or they can be picked up at community organizations. Test kits are also available for purchase online from the manufacturer (bioLytical laboratories) and may be available for purchase at some community pharmacies.
How are self-tests stored and disposed of?
Self-tests need to be stored between 2°C and 30°C. This means that people can keep the test in their home at room temperature. The test is marked with an expiry date, and it must be used before this date. After the test is done, the tester should put all of the components of the testing kit back into the package and throw the package into the garbage.
What role can service providers play in encouraging the uptake of self-testing and supporting people who self-test?
Although self-tests are intended for use by someone on their own, service providers can do a number of things to encourage and support self-testing, including improving awareness of self-testing through educational efforts.
For some people, the cost of a self-test is a barrier to accessing it. If it is feasible, service providers can try to work with partners to provide access to free tests for their community members. Research from other jurisdictions shows that initiatives that allow people to order a free test online can help to reach people who do not test as often as guidelines recommend. Research also shows that efforts to provide clients with tests to distribute to their personal contacts can help to reach first-time testers.
Some organizations may choose to allow people to take a self-test within their facilities (e.g., by making a private room available for self-testing). This may be a good option for people who would benefit from support before and after they take the test. However, service providers should not do the test on a client or interpret the results for them. This is because self-tests are only licensed for a person to perform on themselves. Service providers can help the tester to understand the instructions, which include how to do the test and how to interpret the results.
Unlike with testing that is performed by a healthcare provider or community worker, there is no requirement for a person who is accessing self-testing to receive counselling. However, service providers can support people who decide to self-test. Service providers should be prepared to answer questions about self-testing and help people to decide if self-testing might be right for them. It is important to make people aware that the self-test is a screening test and that confirmatory testing will be needed in the event of a reactive result. Clients should also be made aware of the window period of the test.
After a person has taken the test, service providers can play a role in linking them to appropriate services. Whether a person’s test result is positive or negative, HIV testing is an important entry point for people into other services, such as HIV care, treatment and prevention, as well as other services such as harm reduction and housing.
Service provider resources
HIV testing technologies – fact sheet
HIV transmission – fact sheet
Client resources
whereto.catie.ca – online directory showing where people can access self-tests
HIV Self-Testing – webpage
HIV Basics – brochure
I Know My HIV Status – brochure
HIV Testing: What You Need to Know – video
HIV Testing: Everything You Need to Know – booklet
Seven Ways to Prevent HIV – booklet
INSTI HIV Self Test Training Video – video by bioLytical Laboratories
References
- World Health Organization. Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. Geneva: World Health Organization; 2016. Available from: https://apps.who.int/iris/bitstream/handle/10665/251655/9789241549868-eng.pdf?sequence=1&isAllowed=y
- Taylor D, Durigon M, Davis H, et al. Probability of a false-negative HIV antibody test result during the window period: a tool for pre-and post-test counselling. International Journal of STD & AIDS. 2015 Mar;26(4):215-24.
- Steehler K, Siegler AJ. Bringing HIV self-testing to scale in the United States: a review of challenges, potential solutions, and future opportunities. Journal of Clinical Microbiology. 2019;57(11):e00257-19.
- Galli RA, Lo Hog Tian JM, Sumner-Williams M, et al. An observed, prospective field study to evaluate the performance and acceptance of a blood-based HIV self-test in Canada. BMC Public Health. 2021;21(1):1421. Available from: https://link.springer.com/content/pdf/10.1186/s12889-021-11418-z.pdf
- bioLytical Laboratories. INSTI® HIV Self Test Instructions for Use. 2020.
- Geenius™ HIV 1/2 Confirmatory Assay. A qualitative assay for the confirmation and differentiation of individual antibodies to HIV-1 and HIV-2 in whole blood, serum, or plasma specimens. Marnes-la-Coquette, France: Bio-Rad; 2013. Available from: http://www.bio-rad.com/webroot/web/pdf/inserts/CDG/en/883601_EN.pdf
- Witzel TC, Bourne A, Burns FM, et al. HIV self-testing intervention experiences and kit usability: results from a qualitative study among men who have sex with men in the SELPHI (Self-Testing Public Health Intervention) randomized controlled trial in England and Wales. HIV Medicine. 2020;21:189-97.
- Figueroa C, Johnson C, Verster A, et al. Attitudes and acceptability on HIV self-testing among key populations: a literature review. AIDS and Behavior. 2015 Nov 1;19(11):1949-65.
- Figueroa C, Johnson C, Ford N, Sands A, Dalal S, Meurant R, Prat I, Hatzold K, Urassa W, Baggaley R. Reliability of HIV rapid diagnostic tests for self-testing compared with testing by health-care workers: a systematic review and meta-analysis. Lancet HIV. 2018;5:e277-e290.
Author(s): Harrigan M
Updated: 2023