In 2016, the World Health Organization (WHO) released the Global Health Sector Strategy on Viral Hepatitis, 2016–2021.1 The goal of the strategy is to eliminate viral hepatitis as a major public health threat by 2030. This is the first global strategy on viral hepatitis and it lays out the targets and approaches we need to meet this ambitious goal.
Canada has signed on to this strategy.2 Achieving the strategy’s targets will require important changes in the Canadian hepatitis response. We all have a role to play. This article will detail the strategy targets, examine how Canada is doing in these target areas, and discuss some key shifts in hepatitis C programs and policies that will be necessary to achieve these targets by 2030.
What is the global strategy on viral hepatitis and why is it important?
A global health sector strategy on viral hepatitis is critical considering the scale of the hepatitis pandemic, as well as the limited and fragmented response at the national and global levels. The viral hepatitis pandemic world-wide is responsible for an estimated 1.4 million deaths per year from acute infection and hepatitis-related liver cancer and cirrhosis.1 Hepatitis B accounts for 47% of these deaths and 48% are attributed to hepatitis C.1
The WHO strategy is the first global health strategy on viral hepatitis (hepatitis A, B, C, D and E). The strategy focuses particular attention on hepatitis B and C because of the relatively large public health burden they present.
The strategy asserts that ending the hepatitis pandemic is feasible. The strategy’s vision is “A world where viral hepatitis transmission is halted and everyone living with viral hepatitis has access to safe, affordable and effective prevention, care and treatment services.”1 Aside from setting a specific vision, targets and priority actions for the global community, the strategy also details some programming and policy interventions needed to successfully achieve its targets. These interventions will be discussed later in the article. The strategy provides a strong road map and clear direction to all those engaged in the hepatitis response.
What are the global strategy targets and how is Canada doing in these areas?
The strategy outlines five strategic directions and sets global targets to help shape national goals and targets. The first strategic direction calls for each country to collect data on the epidemic at the national level and to develop specific national targets and an evidence-based national hepatitis plan.
Despite having signed on to the global strategy, Canada does not currently have a national hepatitis strategy and has not yet developed specific national targets.
Although Canada has not developed specific national targets, the following global targets outlined in the strategy provide a starting place to examine how Canada is doing in some of the impact and service coverage targets:
Impact targets
- Incidence: By 2030, there will be a 90% reduction of new cases of chronic viral hepatitis B and C infections.
There is no data available on the number of new cases (incidence) of hepatitis B and C in Canada required to monitor this WHO target; however, Canada does collect data on new diagnoses. This data will be used to examine how Canada is doing, but it should be noted that this number includes the diagnoses of both new and existing infections and is not a true measure of incidence.
Between 2005 and 2014, the rate of reported diagnoses of hepatitis C decreased steadily from 40.2 per 100,000 people to 29.3 per 100,000. This represents a drop from 12,990 diagnoses in 2005 to 10,458 in 2014.3 To accomplish a 90% reduction in the number of new hepatitis C diagnoses by 2030, we would have to see a drop to 1,046 diagnoses.
A total of 302 new acute hepatitis B diagnoses were reported in 2005, corresponding to an overall rate of 1.0 per 100,000, compared to 178 new diagnoses in 2014 (a rate of 0.5 per 100,000). To accomplish a 90% reduction in the number of new acute hepatitis B diagnoses by 2030, we would have to see a drop to 18 diagnoses.
There were 752 diagnoses of chronic hepatitis B reported in 2005, representing a rate of 5.9 per 100,000, which increased to a high of 4,634 diagnoses in 2012 (a rate of 13.6 per 100,000).3 Since then, the number of reported diagnoses has declined to 4,058 in 2014 (a rate of 12.0 per 100,000). To accomplish a 90% reduction in the number of new diagnoses of chronic hepatitis B by 2030, we would have to see a drop to 406 diagnoses.
- Mortality: By 2030 there will be a 65% reduction in mortality rates
In 2013, no deaths from acute hepatitis C infection were reported in Canada (as compared to 33 in 2012); however, 465 deaths were attributed to chronic hepatitis C infection.3 It is likely that there is considerable underestimation of the number of deaths related to hepatitis C because of potential misclassification on death certificates. To accomplish a 65% reduction in deaths related to hepatitis C by 2030, we would have to see a drop to 163 deaths.
In 2013 (the most recent year for which mortality data were available), acute hepatitis B infection was documented as the leading cause of death in 18 people in Canada, and a further 50 deaths were attributed to chronic hepatitis B.3 Initial observations on mortality data suggest a rising number of deaths attributable to chronic hepatitis B infection between 2011 and 2013. The true magnitude of deaths related to hepatitis B is also likely higher due to potential misclassification on death certificates.3 To accomplish a 65% reduction in hepatitis C related deaths by 2030, we would have to see a drop to 24 deaths.
Service coverage targets
- Prevention: By 2030, there will be an average of 300 sterile needles and syringes provided per person who injects drugs per year.
Coverage estimates for Canada estimate that 148 needles/syringes are distributed per person who injects drugs.4 There are no needle and syringe programs operating inside prisons in Canada.5 Based on this data, Canada will have to double the number of needles distributed per injector per year to meet the target.
- Prevention: By 2030, there will be 90% childhood hepatitis B vaccine coverage
In Canada’s provinces and territories, the hepatitis B vaccine is administered either as part of routine infant immunization or through school-based programs. For jurisdictions with a three-dose hepatitis B infant immunization program, the coverage estimate was 75% by age seven. By 17 years of age, an estimated 88% of Canadian children had at least one dose of the three-dose hepatitis B vaccine.6 As we continue to make progress in vaccination coverage for hepatitis B, this target may be realistic for Canada to meet by 2030.
- Prevention: By 2030, 100% of blood donations are screened in a quality-assured manner
Blood donations in Canada are routinely screened for HIV, hepatitis B, hepatitis C and other blood-borne pathogens. There have been no recent transmissions of these infections by blood transfusions.7 Canada has met this target.
- Prevention: By 2030, 90% coverage of hepatitis B virus birth-dose vaccination or other approach to prevent mother-to-child transmission
It is recommended that all pregnant women in Canada are offered a test for hepatitis B during prenatal visits or at the time of delivery.8 All infants born to mothers with hepatitis B are offered the hepatitis B vaccine.8 While no estimates are available to measure whether Canada has met this target, there are systems in place to meet it by 2030.
- Prevention: By 2030, 90% of injections will be administered with safety-engineered devices in and out of health facilities
This indicator related to injection safety is more relevant in low-and middle-income countries where there is an ongoing risk of hepatitis transmission through the medical system. In Canada, most injections are provided with a new single-use syringe and re-use is extremely rare. However, there is a residual risk of hepatitis transmission to healthcare workers through needlestick injuries, which is why safety-engineered devices are recommended.
Safety-engineered devices have built-in safety controls that reduce and potentially prevent needle stick injuries for healthcare workers taking blood samples or through other injections. In Canada, regulations relevant to the prevention of needle stick injuries are developed by provincial authorities. Not all provinces have passed legislation mandating the use of safety-engineered devices. For Canada to achieve the WHO target, all provinces and territories should consider the use of safety-engineered devices in and out of health facilities.
- Testing: By 2030, 90% of people living with hepatitis C and hepatitis B will know their status
According to estimates from the Public Health Agency of Canada, an estimated 44% of people infected with hepatitis C do not know they have hepatitis C (and an estimated 54% do not know they have hepatitis B).9 This tells us that we have a long way to go to meet the target of 90% by 2030. There needs to be increased uptake of new testing technologies, public awareness campaigns and innovative approaches to meet this target.
- Treatment: By 2030, 80% of all eligible persons with chronic hepatitis B or C will be treated.
According to a 2013 report by the Canadian Liver Foundation, less than 10% of people with hepatitis B and less than 25% of people with hepatitis C have been treated.10 However, now that highly effective and short course direct-acting antiviral (DAA) therapies are available for the treatment of hepatitis C and are on formularies across Canada, the number of people receiving hepatitis C treatment should increase. This current level of treatment coverage tells us that we have a long way to go to meeting the target of 90% for both hepatitis B and C by 2030.
Monitoring Canada’s Progress
It is crucial that Canada starts to systematically collect and monitor national-level data to show progress towards these indicators in an ongoing fashion. Currently these data do not exist in any systematic or coordinated fashion.
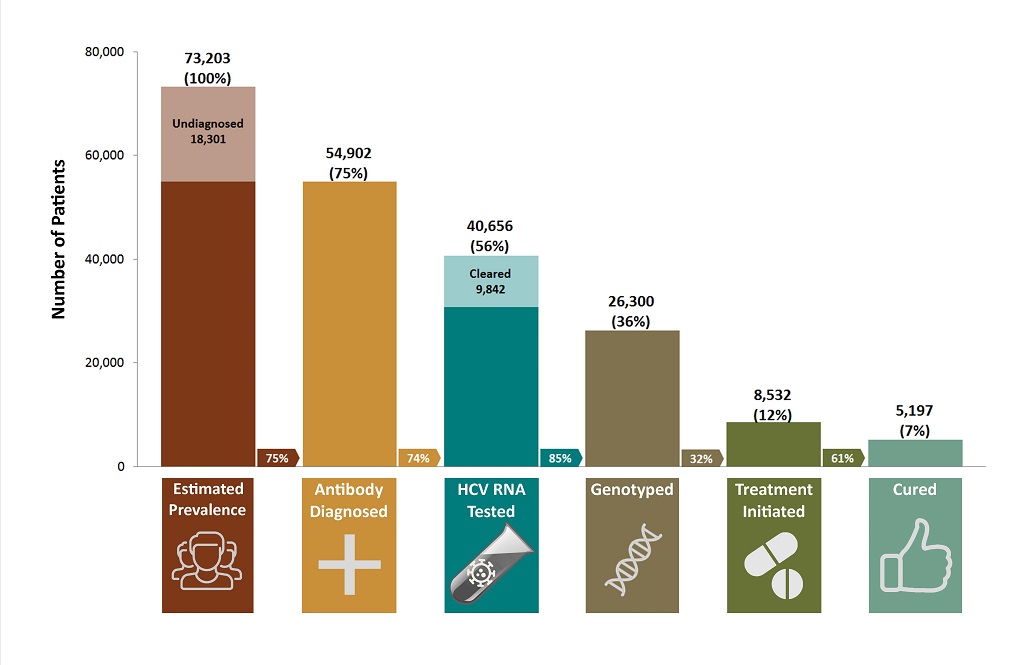
In terms of assessing where Canada is at currently and what else is needed to achieve the WHO global strategy targets, the most comprehensive data and modelling come from British Columbia. British Columbia has developed a cascade of hepatitis C care11 using population-level data from the B.C. Hepatitis Testers Cohort. The cascade of care demonstrates the numbers of individuals with hepatitis C who get tested and subsequently move through the continuum of hepatitis services towards retention in care, treatment and cure.11
The B.C. cascade is likely a relevant snapshot of how Canada is currently doing at each stage of the continuum, and signals where there are gaps. Notably, the WHO global strategy uses the cascade of care as an organizing framework to underscore the specific actions necessary to achieve the global targets.
From the B.C. cascade of care for 2012, shown in Figure 1, it is evident that treatment uptake is particularly low. Only 56% of the estimated number of people living with hepatitis C in B.C. have had a confirmatory RNA test, only 12% have started treatment, and only 7% have been cured. The cascade was developed during the interferon era of treatment, so it is expected that treatment uptake will increase in the coming years as treatment is easier to take and of a shorter duration.
Figure 1. The B.C. hepatitis C cascade of care (2012)
Programming and policy implications
To achieve the global viral hepatitis targets laid out in the WHO strategy, important programmatic and policy shifts need to happen in Canada. The WHO strategy outlines broad priority actions to be taken by each country and the WHO. The proposed actions are intended to guide country efforts, with countries defining and implementing specific actions that are most appropriate to their respective hepatitis epidemics.
This section utilizes the Canadian national-level hepatitis C data, as well as the more specific B.C. cascade of care data to tease out some key actionable steps for Canadian hepatitis C service providers and policy makers.
Hepatitis C prevention
In Canada, the hepatitis C epidemic is most prevalent amongst people who use injection drugs.12 The WHO strategy has several priority actions related to harm reduction. It recommends that countries implement a comprehensive package of harm reduction services, integrate hepatitis C services into harm reduction programs, and address legal and institutional barriers to the provision of harm reduction services.
The data suggests that Canada has a long way to go to reach optimal comprehensive harm reduction coverage, including meeting the target of 300 new needles for every person who injects drugs.
Despite ongoing barriers to providing comprehensive harm reduction services across Canada, it is important that organizations consider the following approaches:
- Provide a comprehensive package of harm reduction services and programming including unlimited provision of sterile needles and syringes, as well as other drug equipment such as crack pipes; access to opioid substitution therapy; risk reduction education; and broad drug user health services as recommended in the Best Practice Recommendations for Canadian Harm Reduction Programs.13
- Provide clear programming guidelines on how to improve provision of harm reduction services,13 including how to tailor harm reduction services to local context and communities being served, ensuring culturally relevant programming for priority populations, and expanding programming to rural and remote areas.
- Link and integrate hepatitis C services within harm reduction and community health services.
- One of the most significant challenges to accessing services is existing laws and policies, which criminalize drug use, drug possession and ultimately people who use drugs. One of the recommendations made in the global strategy is to address legal and institutional barriers to the provision of harm reduction services. Addressing the de-criminalization of drug use is a policy change that might be considered.
Hepatitis C testing
The WHO strategy calls for a high diagnoses rate for hepatitis C. A related priority action outlined in the strategy is to integrate viral hepatitis testing into national hepatitis policies and guidelines that define priority populations, as well as testing locations and strategies.
Broadening public education and testing efforts in Canada is essential. It is also critical to ensure that once people are tested, they are linked with care and treatment options. Canadian testing guidelines do not recommend age cohort testing, which misses a significant proportion of the undiagnosed population in Canada.14 Despite these limitations, wherever possible the following approaches can be useful to increase front-line testing:
- Partner with other organizations and services to help enable seamless and timely linkage to testing, treatment and care.
- Provide health navigation support to clients who have been tested at one site and are being linked to care at another organization.
- Expand public and tailored education and testing efforts, including offering hepatitis C testing in new sites frequented by priority populations, including harm reduction programs, shelters, newcomer programs, community centres, and as part of testing fairs.
- Integrate new testing technologies and approaches into practice such as dried blood spot testing and point of care testing.
Hepatitis C treatment
The WHO target of providing treatment to 80% of all eligible persons by 2030 will require important programming and policy shifts in Canada. The most significant gap along the continuum of care in Canada is treatment uptake.11 The availability of highly effective oral therapies on provincial and federal formularies across Canada creates an opportunity to substantially increase treatment roll out. The key will be to make these treatments accessible to priority populations, as well as making re-treatment accessible in the case of re-infection. Concerns about re-infection are often cited as a reason for not offering treatment to individuals at high risk of re-infection, yet there is actually strong evidence to suggest that offering treatment to the most high-risk individuals is instead the most effective approach to achieving elimination of hepatitis C at both the individual and population level.15
Several ways this can be achieved include:
- In the case of re-infection, offer re-treatment without discrimination.
- Utilize ‘bring a friend’ strategies within organizations offering treatment, to increase treatment scale-up within high-risk sub-populations.
- Expand the reach and scope of hepatitis C treatment programs to underserved priority populations and target small local networks with a high prevalence of hepatitis C. This can include providing comprehensive harm reduction services to prevent re-infections.16
Health equity across the hepatitis C continuum of care:
Delivering for equity is another one of the five strategic directions in the WHO strategy. Structural barriers such as ‘poverty, discrimination and criminalization, drug dependence and poor mental health1 increase vulnerability and prevent equitable access to hepatitis C services. In Canada, stigma, racism and discrimination within the healthcare system can deter certain populations from accessing services – in particular Indigenous peoples, people who use drugs, and other stigmatized groups.17 When marginalized and stigmatized populations do access services, the services can be culturally or structurally inappropriate and ineffective at serving those populations. The WHO strategy calls for the creation of institutional and community environments that make it safe for people to access hepatitis services, which includes involving people most affected in the planning and delivery of services. It is also critical that hepatitis services address the social factors, such as poverty, trauma and stigma, that can impact participation in sexual and drug use practices that place people at greater risk of infection.
Activities that can increase access and relevance of services for priority populations include:
- Involving people with lived experience of hepatitis C and priority populations in all stages of program planning and delivery.
- Developing or partnering with services provided by and for communities most affected by hepatitis C.
- Providing or referring to social services as part of the hepatitis C continuum of care including employment, housing, mental health, and addiction and harm reduction services.
References
- a. b. c. d. e. World Health Organization. Global health sector strategy on viral hepatitis, 2016–2021. 2016 Jun. Available from: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1
- Canadian Society for International Health. Canada takes a stand against Viral Hepatitis. [Online]. Available from: http://www.catie.ca/en/news/canada-takes-stand-against-viral-hepatitis
- a. b. c. d. e. Public Health Agency of Canada. Report on Hepatitis B and C in Canada: 2014. Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada. 2017. Available from: https://www.canada.ca/en/services/health/publications/diseases-conditions/report-hepatitis-b-c-canada-2014.html
- Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Global Health. 2017;5(12):e1208–e1220.
- van der Meulen E, Claivaz-Loranger S, Clarke S, et al. On point: Recommendations for prison-based needle and syringe programs in Canada. 2016 Jan. Toronto, ON. Available from: http://www.aidslaw.ca/site/on-point-recommendations-for-prison-based-needle-and-syringe-programs-in-canada/?lang=en
- Public Health Agency of Canada. Vaccine coverage in Canadian children: Results from the 2013 Childhood National Immunization Coverage Survey (CNIS). 2016. Available from: http://publications.gc.ca/collections/collection_2016/aspc-phac/HP40-156-2016-eng.pdf
- Canadian Blood Services. Surveillance report. 2016. Available from: https://www.blood.ca/sites/default/files/Surveillance_Report_2016_EN.pdf
- a. b. National Advisory Committee on Immunization (NACI). Hepatitis B vaccine. Canadian Immunization Guide, Seventh Edition. Ottawa: Canadian Medical Association, 2006.
- Rotermann M, Langlois K, Andonov A, Trubnikov M. Seroprevalence of hepatitis B and C virus infections: Results from the 2007 to 2009 and 2009 to 2011 Canadian Health Measures Survey. Statistics Canada`s Health Reports. 2013 Nov; 24(11). Available from: http://www.statcan.gc.ca/pub/82-003-x/2013011/article/11876-eng.htm
- Canadian Liver Foundation. Liver disease in Canada: A crisis in the making. 2013 Mar. Available from: https://www.liver.ca/wp-content/uploads/2017/09/CLF_LiverDiseaseInCanada_Synopsis_E.pdf
- a. b. c. Janjua NZ, Kuo M, Yu A, et al. The population level cascade of care for hepatitis C in British Columbia, Canada: The BC Hepatitis Testers Cohort (BC-HTC). EBioMedicine. 2016;12:189–195.
- Trubnikov M, Yan P, Archibald C. Estimated prevalence of hepatitis C virus infection in Canada, 2011. Canada Communicable Disease Report. 2014 Dec18;40(19). Available from: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/14vol40/dr-rm40-19/surveillance-b-eng.php
- a. b. Strike C, Hopkins S, Watson TM, et al. Best practice recommendations for Canadian harm reduction programs that provide service to people who use drugs and are at risk for HIV, HCV, and other harms: Part 1. 2013. Toronto, ON: Working Group on Best Practice for Harm Reduction Programs in Canada.
- Canadian Task Force on Preventive Health Care. Recommendations on hepatitis C screening for adults. Guidelines. CMAJ. 2017 April 24:189(16):E509–604. Available from: http://www.cmaj.ca/content/189/16/E594
- Midgard H, Weir A, Palmateer N, et al. HCV epidemiology in high risk groups and the risk of reinfection. Journal of Hepatology. 2016 Oct;65(1 Suppl):S33–S45.
- Islam N, Krajden M, Shoveller J, et al. Incidence, risk factors, and prevention of hepatitis C reinfection: a population-based cohort study. The Lancet Gastroenterology & Hepatology. 2017 March;2(3):200–210.
- Allan B. & Smylie J. First Peoples, second class treatment: The role of racism in the health and well-being of Indigenous peoples in Canada. 2015. Toronto, ON: The Wellesley Institute. Available from: http://www.wellesleyinstitute.com/wp-content/uploads/2015/02/Summary-First-Peoples-Second-Class-Treatment-Final.pdf
About the author(s)
Suzanne Fish works at CATIE as the knowledge broker in hepatitis C, community health programming. Suzanne has an M.A in Political Economy and has spent the past 10 years engaged in health equity and community engagement initiatives with a range of social service agencies, community organizations and grassroots civil society groups around the globe. She has focused her research and social justice work on centring insights from lived experience and evidence-based interventions within project, program and policy-development arenas.
Melisa Dickie is Associate Director, Hepatitis C Knowledge Exchange at CATIE. She also serves on the steering committee of Action Hepatitis Canada.

